These posters have been presented at meetings in Children's Mercy and around the world. They represent research that was done at the time they were created, and may not represent medical knowledge or practice as it exists at the time viewers access these posters.
-

Clinical Course of a Patient With Agammaglobulinemia Caused by SLC39A7 defect
Thao Le, Emily Farrow, Alvin Singh, Isabelle Thiffault, and Nikita Raje
Case Report: A 10-year-old unimmunized boy initially presented to the hospital at 18-months of age with pneumonia and failure to thrive. He had multiple infections including Escherichia coli urosepsis, viral croup, chronic otitis media with bilateral ruptured tympanic membranes, and bacterial pneumonia. On physical examination, he was ill appearing and had diffuse crackles. His laboratory work-up showed leukocytosis, normocytic anemia, undetectable immunoglobulin (Ig) G, A, and E, low IgM (28 mg/dL), absent B cell with normal T cell (7800 mm3) and NK cell (527 mm3) counts, and low zinc level (63 mcg/dL). Genetic testing was negative for Bruton tyrosine kinase (BTK). Ig replacement therapy (IgRT) was initiated. Despite therapy he had persistent chronic rhinosinusitis and chronic cough. Computed tomography (CT) scan of the sinus and chest showed pansinusitis and bronchiectasis with mucoid impaction, predominantly in the lower left lobe. He was treated with inhaled corticosteroids and chest physiotherapy. He underwent functional endoscopic sinus surgery and bronchoscopy, which showed non-typeable Haemophilus. He was then treated with a course of Augmentin. He also consistently grew below the 3rd percentile while his mid-parental target height is close to the 50th percentile. He was started on growth hormone and has responded well. Exome sequencing showed compound heterozygous variants in the SLC39A7 gene encoding the zinc transporter, ZIP7. This leads to an autosomal recessive agammaglobulinemia-9, a primary immunodeficiency syndrome with recurrent bacterial infections associated with agammaglobulinemia and absence of circulating B cells. The family declined prophylactic antibiotics despite persistent infections. At 10 years of age, he continued to have worsening chronic sinusitis and bronchiectasis. A trial of prophylactic antibiotics with Amoxicillin was initiated. Discussion: Our patient is a 10-year-old male with compound heterozygous variants in SLC39A7 presenting with agammaglobulinemia, short stature, and bronchiectasis. He continued to have chronic recurrent sinopulmonary infections despite therapeutic IgG level on IgRT. Starting prophylactic antimicrobials earlier may be helpful in reducing lung injury and preventing infections
-
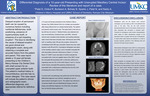
Clinical Presentation and Differential Diagnosis of a 10-year-old Presenting with Unerupted Maxillary Central Incisor: A Case Report.
Dev Patel, Robin Onikul, Amy Burleson, Brenda S Bohaty, Jenna Sparks, Neena Patel, and A Naidu
Delayed eruption of permanent teeth can be caused by numerous factors including nutritional deficiencies, tooth positioning, presence of supernumerary tooth, or presence of a cyst impeding eruption. The keys to identifying the cause of delayed eruption are good clinical and radiographic exam, along with obtaining a complete and accurate history from the patient and parent. This case report details a 10-year-old female presenting to the Children’s Mercy Kansas City Dental Clinic with chief concern for an unerupted upper anterior tooth. The patient’s medical history is noncontributory, and she has no known allergies. This case report will include the patient’s clinical and radiographic exam, differential diagnosis, and appropriate referral for definitive care.
-

Comparative Study of Pain Perception with Use of Vibration and/or Cold Stimulation Applied During Local Anesthetic Delivery in a Dental Setting: A Systematic Review
Tara Craven, Gage Williams, and Brenda S Bohaty
Delivery of local anesthetic can be one of the most difficult parts of the procedure for pediatric patients undergoing dental treatment and can prevent the child from being able to cooperate for treatment as well as instill anxiety for future visits.1 Several methods can be utilized to help mitigate pain control during the local anesthetic injection including behavior management, vibration, cold sensation, warming the anesthetic, and topical anesthetic.4 These methods are essential to helping the child have a good experience and ultimately be able to tolerate treatment for caries management and a long term positive view of the dentist. One technique that has been previously researched is the use of vibration and cold stimulation when delivering local anesthetic. Vibration and cold stimulation can block the afferent pain fibers (A delta and C fibers), an idea based on the gate control theory, thus reducing pain.11 These two methods, cold stimulation and vibration, can be especially advantageous for a pediatric population because they are both non-invasive. This systematic review is aimed at reviewing randomized control studies to evaluate the efficacy of using vibration and/or cold stimulation devices while administering local anesthesic in order to lower pain perception and dental anxiety. Several modern devices have been invented to introduce vibration and/or cold stimulation that can be utilized during dental treatment however, research on these devices is limited. Additionally, a systematic review is needed to guide further research as well as a proposed design study to further evaluate the efficacy of using vibration and/or cold stimulation during local anesthetic delivery.
-

Drug metabolizing enzymes and transporters may help determine effective budesonide dosing in EoE
Laurie McCann, Lisa Harvey, Norah Almahbub, Wendy Y. Wang, Erin C. Boone, Janelle R. Noel-Macdonnell, and Rachel Chevalier
Background: Eosinophilic esophagitis (EoE) is a chronic inflammatory disorder diagnosed in children with painful or difficult swallowing, vomiting, or poor weight gain. Current treatment models adopt a trial-and-error approach in regard EoE treatment, including restrictive elimination diets, proton pump inhibitors, and topical budesonide. This approach can delay effective treatment which increases risk of disease progression and increases medical costs to families for frequent clinic visits and endoscopy. Objectives/Goal: The objective of this study is to determine the CYP3A5 genotype and expression of patients with eosinophilic esophagitis to discover which patients will respond to standard dosing of topical budesonide treatment (1, 2). Budesonide is a known drug substrate of CYP3A5 protein and single polymorphic changes are known to affect drug metabolism. By determining the CYP3A5 genotype, we aim to correlate treatment response to topical budesonide treatment, leading to more targeted and individualized dosing of budesonide. Allelic variant *1 (wild-type) has shown high substrate metabolism (1). Allelic variant *3 (most common), *6 , *7 have shown reduced substrate metabolism (1). The ultimate goal of this study is to aid in the development of a simple serum test to check a patients’ CYP3A5 genotype at the time of EoE diagnosis, prior to initiation of topical budesonide. Methods/Design: This is a single center retrospective study ongoing at Children’s Mercy Hospital in Kansas City, Missouri using serum and esophageal tissue samples from the already established, ongoing Gastroenterology Repository for Information in Pediatrics biorepository (GRIP) from patients <20 years>old, enrolled from 8/1/2017 to 11/1/2022. For this interim analysis, we had samples from 22 patients for genotyping and mRNA extraction. Digital droplet PCR (ddPCR) was used for mRNA quantification. Results: Of the 22 samples, nineteen had *3/*3 CYP3A5 alleles (86.3%), two had *1/*1 alleles (9%), and one had *1/*3 alleles (4.5%). CYP3A5 ratios were performed, and the initial results of this small sample size so far show that expression does not vary between genotype. Compared to CYP3A4, there is more CYP3A5 expression in the esophagus than CYP3A4, as noted in prior studies in adults. ddPCR was able to successfully measure CYP3A5 expression in esophageal biopsies confirming this as a valuable tool to quantify mRNA in these small tissue samples. Conclusions: These initial results are consistent with already published data and will help lay the groundwork for larger, more in-depth studies. We anticipate more data forthcoming, as the collection of consented patients is ongoing. Future, larger prospective studies are needed to further propagate the development of precision therapeutics of budesonide in EoE patients.
-

My Global Health Experience - Eldoret, Kenya
Megan Carroll
Describes her experience in Eldoret as a 4th year medical student.
-

Practicing Pediatrics in Lake Atitlan, Guatemala
Chandra Swanson
Describes her experience working in Lake Atitlan, Guatemala.
-
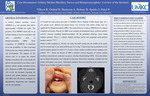
Solitary Median Maxillary Incisor and Holoprosencephaly: A review of the literature
Benjamin Olson, Robin Onikul, Amy Burleson, Brenda S. Bohaty, Jenna Sparks, and Neena Patel
A solitary median maxillary incisor (SMMCI) is a rare anomaly that affects approximately 1:50,000 live births. The presence of an SMMCI tooth may be an isolated finding or may be associated with other anomalies such as Holoprosencephaly (HPE). Early recognition of SMMCIS is important given the possibility of other associated findings that may impact pediatric growth and development. The purpose of this clinical case is to report the clinical findings associated with a nine-month-old patient who presented to our clinics with a primary solitary median maxillary incisor and a diagnosis of HPE. This presentation will review the literature regarding the medical and dental findings associated with HPE and will discuss strategies for appropriately diagnosing and managing care for such patients.
-

Who’s my child’s main doctor? Caregiver Perceptions of Teaching Teams
Jonathan Ermer, Shelby Chesbro, Jessica Boerner, Jacqueline M. Walker, and Joy L. Solano
Background: At academic medical centers, children are cared for by large medical teams consisting of multi-level learners including medical students, interns, senior residents and fellows on patient-and family-centered rounds (PFCR). The size and structure of these teaching teams can make it difficult for patients and families to know who is caring for them and establish a trusting relationship. Also, research has shown when residents are perceived as a “team leader,” they learn more. Previous literature in adults has shown that patients often perceive the intern as their “main doctor,” but this has not been studied in pediatrics. Objective: We sought to identify who caregivers identify as their child’s main doctor and recorded the frequency of caregivers who identified the presenter (medical student or intern) as their child’s main doctor. Additionally, we described the frequency of caregivers who were aware that their child was going to be cared for by a large team of doctors at different levels of training. Methods: We conducted a single-institution prospective cohort study using a convenience sample of caregivers admitted to the hospital on pediatric hospital medicine teaching teams that conduct PFCR. After a rounding encounter, we surveyed caregivers who speak English or Spanish. Masked photos of all team members were provided to caregivers when asked to identify the child’s main doctor and the leader of the team. For each patient with permission from the caregiver, we recorded the number of prior admissions, active consultants during the current admission, and number of complex chronic care conditions. Results: A total of 99 patient caregivers were surveyed. Patient demographics are listed below: 46% of respondents did not know their child would be cared for by a large team of doctors, and 43% of respondents did not know learners would be involved in their child’s care. Graphs below indicate who caregivers selected as their main doctor, and in charge of their child’s care team. Discussion: In this study, caregivers varied in who they thought was their child’s main doctor and the leader of their child’s care team. Most were unaware that large medical teams with learners at different levels of their training would care for their child. Work can be done to better orient families and caregivers to teaching teams. This could include a standardized introduction process on admission within each hospital system.
-

A Metabolic, Mechanical, Multi-Organ Masterpiece: Dural Device Support Bridge to En-Bloc Heart-Liver Transplantation in Propionic Acidemia
Rebecca Juhl, Brian Birnbaum, Aliessa P. Barnes, William Gibson, Bhargava Mullapudi, Beth Lang, Megan Faseler, Daniel E. Heble, Victoria Urban, Ryan T. Fischer, Jennifer L. Gannon, and David Sutcliffe
Introduction: Propionic Acidemia (PA) is a disorder related to abnormal protein and lipid metabolism resulting in progressive neurological injury and dilated cardiomyopathy (DCM). Interventions for PA and secondary disease manifestations can require multi-organ transplantation. Herein we report the case of a child with PA and end-stage DCM requiring left ventricular assist device (LVAD) support with eventual heart-liver transplant. Case Report: A 17 year old male diagnosed in childhood with PA developed chronically progressive DCM culminating in end stage heart failure with acute decompensations. In a recurrent admission, he progressed to require dual inotropic support and systemic anticoagulation for new LV thrombus. Heart-liver transplant eligibility was confirmed, and with further clinical deterioration requiring invasive mechanical ventilation and chemical paralysis he underwent durable, intrapericardial LVAD implant as bridge to candidacy. After accomplishing discharge, he achieved intense physical and metabolic-specific nutritional rehabilitation over the span of 3 months, and he was listed for combined heart-liver transplant. In preparation for the complexities related to dual organ transplant from LVAD support, collaborative operative simulations were completed as were adaptations of post operative immunosuppressive and medical management between transplant teams. After a 3 week waitlist duration, he underwent en-bloc heart-liver transplant with successful post operative recovery to discharge within 2 weeks. Summary: Even with effective and specialized nutritional management, DCM secondary to PA can progress to end stage heart failure requiring mechanical support and organ transplantation. Successful rehabilitation via VAD and bridge to multiorgan transplantation requires diligent collaboration across transplant teams. Despite both technical and clinical challenges, successful completion of en-bloc heart-liver transplant from VAD support can be accomplished.
-
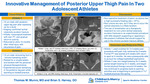
Innovative Management of Posterior Upper Thigh Pain In Two Adolescent Athletes
Thomas Munro and Brian Harvey
Case history: Two 16-year-old males with posterior upper leg pain. Athlete A reports he had been exploding up to dunk a basketball when he felt a pop with immediate pain in his posterior LLE. He was treated conservatively with activity modification and PT resulting in improvement of symptoms and return to full activity. He returned 15-months after the initial injury due to recurrence of pain. Athlete B stated he had tweaked his hamstring multiple times over the summer and had been working with a physical therapist for rehabilitation. He had attempted full rest for a couple weeks with graded return to activity. He had return to full activity before experiencing a repeat event of severe pain in his posterior upper leg while running at full speed in football. Athlete B stated that if felt like his leg suddenly gave out on him and lost strength. He denies hearing a specific pop. Physical Exam: Athlete A and Athlete B both have normal appearing skin and musculature of the posterior thigh. Both athletes have limited passive extension of the LLE and decreased LLE strength, most significant with resisted knee flexion. Athlete A and B both have focal pain over the left ischial tuberosity with athlete B reporting tenderness extending slightly into the proximal hamstring. No pain is reported when palpating over the ASIS, AIIS, pelvic crest, or greater trochanter. Both athletes have a negative FABER, FABER, and Log Roll. Athlete A has been using crutches to assist with ambulation. Athlete B has a slightly antalgic walking pattern with reported discomfort/pain in left upper extremity. Differential diagnosis: 1) Proximal hamstring strain 2) Ischial tuberosity avulsion fracture/apophysitis 3) Piriformis syndrome 4) Stress fracture (Ischial or femoral) 5) Ischial femoral impingement syndrome Tests and results: - Athlete A: Xray: Left ischial apophysis avulsion fracture. CT: redemonstration of an apophyseal avulsion fracture (1.5cm displaced) with interval development of calcified callus/heterotopic ossification. CT guided fenestration. - Athlete B: Xray: asymmetric appearance the ischial apophyses, with widening/irregularity on the left. MRI Pelvis: Inflammatory marrow signal of the left ischial apophysis. Mild edema around the proximal hamstring tendons. CT guided fenestration. Final/Working Diagnosis: Athlete A: Chronic avulsion fracture of the ischial tuberosity with subsequent nonunion. Athlete B: Acute on chronic ischial tuberosity avulsion fracture. Discussion: There is controversary over the best treatment strategies for pelvic avulsion fractures. In general, non-operative treatment of fractures with < 2cm displacement ( < 1.5 cm for ischial fractures) have a high success rate resulting in healing about 97% of the time. Non-unions are rare, but if they are to occur the ischial tuberosity is a likely location. The use of fenestration as a therapeutic treatment for non-union ischial tuberosity avulsion fractures has been exhibited in a 3 patient case series and 1 case report. We present two athletes with a chronic/acute on chronic ischial tuberosity avulsion fractures who underwent fenestration of the enthesis of the proximal hamstring. Outcomes: Athlete A had initial successful management with pain improvement, healing changes on imaging, and return to sport. Over a 15-month period he exhibited intermittent pain with worsening acute pain. He subsequently underwent an IR guided fenestration. His pain improved and he demonstrated osseous bridging on imaging. Athlete B also underwent fenestration with concerns of acute on chronic injury.
-
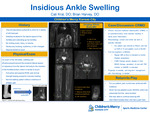
Insidious Ankle Swelling
Catharine Kral and Brian Harvey
10-year-old female presented to clinic for left heel pain and swelling. She participates in dance for an estimated 8-9 hours a week. Symptoms started four weeks prior to presentation. She does not remember any inciting event, fall, trauma, or other mechanism of injury prior to onset. She says the pain is localized to the lateral aspect of her calcaneus and extending up to her Achilles. She has never seen any bruising, erythema, or skin changes overlaying the swollen region. Family reports she intermittently has a limp. It is most tender to touch or when she bumps her foot on something. She continued to dance for the first two weeks, as she says her pain has not worsened with dancing. However, she has tried resting the last two weeks with no improvement in symptoms. She has no pain that wakes her up at night. She denies any numbness or tingling in her foot or toes. The case was discussed with Rheumatology who agreed with CRMO and recommended lab work and additional whole-body MRI. ESR was 15 and platelet count 65,000 while additional MRI showed multiple lesions throughout, including bilateral acetabula, superior/inferior pubic symphysis, right femoral neck, right tibia metaphysis, bilateral tibia epiphysis, right great toe, second and third metatarsals. With the lab work, MRI findings and absence of other type b symptoms, CRMO was the leading diagnosis. NSAIDs were started as the common first line treatment for pain control and disease modification.
-

Phlebotomy-Free Days in Common Conditions Among Hospitalized Children and the Association with Clinical Outcomes
Megan Collins, M Hall, SS Shah, MJ Molloy, PL Aronson, JM Cotter, MJ Steiner, MJ Tchou, JR Stephens, and Jessica L. Markham
Background: Phlebotomy is an invasive procedure associated with pain and iatrogenic anemia. Minimizing phlebotomy for hospitalized children could improve their experience and avoid unnecessary tests. Objective: To describe: 1) the prevalence of phlebotomy-free days among children hospitalized with common conditions and 2) the association of phlebotomy-free days with clinical outcomes. Design/Methods: We performed a multicenter, cross-sectional study of children hospitalized 1/1/2018 to 12/31/2019 with an All Patient Refined Diagnosis Related Group (APR-DRG) for common infections across 38 hospitals in the Pediatric Health Information System (PHIS) database. We excluded patients with length of stay (LOS) < 2 days, medical complexity, interhospital transfers, and those receiving intensive care. We defined phlebotomy-free days (PFDs) as hospital days with no laboratory blood testing and measured the proportion of PFDs per hospital day (PFD ratio) for each condition and hospital. Hospitals were grouped into low, moderate and high average PFD ratios. Adjusted outcomes were compared across groups and included LOS, costs, and all-cause 14- and 30-day readmission rates. Results: We identified 126,135 patient encounters (Table 1). Bronchiolitis (N=31,302), non-bacterial gastroenteritis (N=20,430), and pneumonia (N=16,031) accounted for the greatest number of hospital days. Bronchiolitis (0.78) and pneumonia (0.54) had the highest overall PFD ratios, while bone and joint infections (0.28) and non-bacterial gastroenteritis (0.30) had the lowest overall PFD ratios. There was wide variation across hospitals and conditions in PFD ratios (Figure 1). We identified 8 hospitals with low, 21 with moderate, and 9 with high PFD ratios (Figure 1 and Table 1). There were statistically significant but small differences in the distributions of age, payer, and H-RISK among patients in the low, moderate, and high PFD hospital groups (Table 1). There were no differences in adjusted outcomes across low, moderate, and high PFD hospital groups (Table 2). Conclusion(s): Among children hospitalized with common infectious conditions, there was variation across conditions and hospitals in the proportion of PFDs per hospital day. Hospitals with low, moderate and high ratios of PFDs had no differences in outcomes. Our data suggest at least some laboratory overuse and opportunities to improve the experience and care of children hospitalized with infections.
-

Sepsis-induced Acute Lung Injury and the Development of Bronchopulmonary Dysplasia in Premature Infants
Jacob S. Ward, Hung-Wen Yeh, Megan Tucker, and Venkatesh Sampath
Background: Bronchopulmonary dysplasia (BPD) is a significant cause of morbidity and mortality in premature infants with several known risk factors. Recent literature described the harmful effect of inflammation on the developing lung. Recently, we showed that late-onset sepsis (LOS) and necrotizing enterocolitis (NEC) causes acute lung injury (ALI) in preterm infants as measured by increases in pulmonary severity scores (PSS). The aim of this study was to investigate the association between LOS-induced ALI and BPD outcomes. We hypothesized that LOS would increase the likelihood of developing BPD, and the associated LOS-induced ALI will add to the ability to predict development of BPD. Objectives: 1) Determine if LOS and number of LOS events increase risk of developing BPD. 2) Determine if LOS-induced ALI is more likely to result in BPD. Methods: A retrospective single-center study of 523 infants < 31 weeks gestation and < 1500 grams admitted prior to day of life 7 from 2010-2020 who survived to at least 36 weeks gestation (Fig. 1). Culture positive and rule-out sepsis events were identified, and PSS scores were calculated starting from -72hr from event up to 1 week after the initiation of antibiotics. BPD was defined as per the NICHD (year 2000) consensus definition. Bivariate associations with BPD were assessed by Wilcoxon rank-sum test and Chi-squared test. BPD was predicted by conditional inference forest using demographic plus (1) LOS and (2) plus ALI summary statistics. Results: Among the 523 eligible infants, 198 had at least 1 sepsis event and 325 had no sepsis event. In the sepsis group, 69% developed BPD compared to only 49% of those without sepsis (Fig. 1). Development of BPD showed bivariate associations with sepsis and number of sepsis events (both p < 0.001, standardized mean difference or SMD 0.21 and 0.41, Table 1), the median area under the PSS curves (SMD 0.19, p-value < 0.008), as well as time points +48hr (SMD 0.2, p-value < 0.006), +72hr (SMD 0.2, p-value 0.007), and +168hr (SMD 0.32, p-value < 0.001). Adding sepsis, number of sepsis events, or PSS measures to demographic variables did not increase area under ROC curve in predicting BPD. Conclusions: Our preterm cohort revealed significant bivariate associations of sepsis, number of sepsis episodes, and PSS for BPD. However, they did not improve prediction accuracy of BPD to the demographic variables. Future work will examine the effect of sepsis-induced ALI on severe BPD and use additional parameters such as CRP to enrich our prediction models.
-

A Case of Hexasomy 15q due to a Tricentric Supernumerary Chromosome 15
Emily Farrow, Laura A. Cross, Bonnie Sullivan, Keely M. Fitzgerald, Joseph Alaimo, Elena Repnikova, John Herriges, and Lei Zhang
Background: A 7-month-old male with a history of developmental delay, plagiocephaly, hypotonia, chronic cough/congestion was admitted for abnormal movements. Prolonged EEG revealed focal epilepsy and epileptic spasms. Genetic testing revealed a complex structurally rearranged chromosome 15 which contains two inverted duplicated chromosome 15s joined together at one end, resulting in partial hexasomy for 15q. Case presentation: The proband was born to a G2P2 33-year-old mother following an uncomplicated pregnancy at 40 weeks 2 days gestation. At birth he was 6lbs 8oz, 20in long, and APGARs were 3/5/9 at 1/5/10 minutes. At delivery he was limp, pale and had poor tone with minimal crying and respiratory depression. He was admitted due to persistent seizure-like activity. Aa prolonged EEG was abnormal, but movements were not seizures, and he was discharged without medication. A newborn hearing screen was failed, and follow-up confirmatory testing showed mixed hearing loss with right greater than left. At 7-months-of age he was readmitted for seizure. Repeat EEG was indicative of clinical and subclinical focal seizures and epileptic spasms. At his last evaluation at, 11 months of age, he has global developmental delay, hypotonia, and wears bilateral hearing aids. He is unable to sit unsupported but does have head control, is able to roll over and grabs objects with both hands. Imaging studies to date have been negative, including MRI, echocardiogram, and renal ultrasound. Epilepsy is currently well controlled with medication. Microarray testing was ordered at 7 months and showed six copies of an ~7.6 Mb segment between the common breakpoints BP1 and BP4, followed by four copies of an ~1.5 Mb segment between BP4 and BP5 in proximal 15q from 15q11.2 to 15q13.3. Methylation and copy number analysis of 15q11.2q13.1 for Prader-Willi (PWS) suggested maternal inheritance. Chromosome analysis demonstrated a male karyotype with 47 chromosomes including an extra tricentric chromosome. Interphase FISH analysis shows six copies of SNRPN (15q11.2) in 92% of nuclei and two copies of SNRPN in 8% of nuclei. Metaphase FISH analysis found two enhanced SNRPN (15q11.2) signals consistent with a total of four copies of SNRPN in this additional derivative chromosome, indicating the chromosome 15 is composed of two inverted duplicated 15s linked each other at the ends as a way of mirror image. Parental chromosomes were normal, confirming de novo inheritance. Conclusions: Although supernumerary marker chromosome (SMC) 15 itself is common, occurring in ~1/30,000 births, individuals with a tricentric der(15), resulting in partial hexasomy 15q are rarely reported. Complimentary techniques including microarray, MLPA, FISH, and G-banding were used to resolve the structure of the SMC 15. In the future, novel technologies, such as optical mapping, may also be beneficial in the resolution of complex structural variants.
-

Evaluating the Impact of Long Read Genomes in Rare Disease: A systematic analysis of 1000 HiFi Genomes
Emily Farrow, Isabelle Thiffault, Ana S A Cohen, Tricia N. Zion, Adam Walter, Margaret Gibson, Chengpeng Bi, Warren A. Cheung, Jeffrey J. Johnston, and T Pastinen
Introduction: Genomic technologies continue to advance at a rapid rate, leading to continued novel gene-disease discoveries. However, despite the exponential increase in new gene discoveries, diagnostic rates in rare disease continue to range from 30-50%. To evaluate the impact of long read genome sequencing (lrGS) in a rare disease cohort, lrGS was implemented systematically in an institution-wide research program, Genomic Answers for Kids (GA4K). Methods: Individuals enrolled in GA4K, with a suspected genetic disorder, that remained undiagnosed after exome or genome sequencing, were submitted for HiFi sequencing. Probands were sequenced to a target depth of 30X coverage. Analyses included copy number, structural variation, single nucleotide variation, repeat expansion, and for a subset of genomes 5-methyl C detection. Clinical variants previously reported were used to assess lrGS variant detection algorithms. Additionally, sensitivity and specificity for lrGS were calculated by comparison to an Infinium Global Screening microarray. Results: As we have previously demonstrated, lrGS sensitivity and specificity for SNVs were slightly higher than short read genome sequencing (srGS), at 99%. Additionally, lrGS continued to identify ~150 novel rare variants impacting a coding gene (MAF <0.01%) compared to srGS. Increased coverage and phasing resulted in the detection of variants previously uncalled in sr sequencing, and phasing of variants in singletons, confirming molecular diagnoses. Given the previously demonstrated accuracy of SNV, we next focused analyses on more complex variation, not readily detectable by srGS. Approximately 39% of samples initially screened positive for a potential pathologic expansion (n=59 genes), with filtering criteria maximized for sensitivity. After interpretation, which includes examination of the repeat motif and structure, ~1.6% were considered to be pathogenic alleles, highlighting the importance of sequencing suspected expansions in large cohorts in addition to sizing. When SV/CNV are limited to variants at less than 5% frequency that impact a coding region (CCDS), there are 17.8 variants/genome, of these on average 4 overlap OMIM CCDS. Beyond characterization of coding impact, the nature of SV/CNV allows determination of orientation of duplications (on average 3 rare CCDS duplications per genome) as well as superior detection of infrequent inversions (one in six genomes has CCDS impacting inversion) as compared to other sequencing approaches. Direct 5-methyl-C detection (5mC-HiFi-GS) has been completed in 380 genomes and focusing on rare (< 0.5% population frequency) gene proximal (5’) hypermethylation suggestive of “promoter silencing”, we observed on average 51 such alleles per patient (13 in OMIM genes). To date, two of the OMIM promoter hypermethylation events from 5mC-HiFi-GS are linked to previously undetected pathogenic repeat expansions, but many others are proximal to novel unstable repeats and other non-coding rare variants with potential function. In parallel, the rare methylation signatures faithfully recapitulate previously known disease variant linked epigenetic pertubations (e.g. DM1). Conclusions: The implementation of lrGS in an ES/GS negative cohort resulted in an approximate 10% increase in diagnostic yield. Importantly, previously reported variants were recapitulated, indicating that lrGS could be utilized as a first-tier genome test, simplifying genetic testing algorithms and increasing efficiency. Our developing catalog of rare SVs and methylation variants are now giving new handles for unsolved disease in known and novel disease genes. Anticipated improvements in throughput and cost will enable the widespread integration of long read sequencing into clinical care.
-

If We Know Better, Why Don’t We Do Better? A QI Project Aimed at Addressing Pain Related to Vaccinations
Haley J. Killian, Amanda D. Deacy, Elizabeth Edmundson, Lucy Raab, and Jennifer Verrill Schurman
Introduction: Evidenced-based tools have long existed to combat pain and anxiety associated with needle sticks, yet the gap between knowledge and uptake persists. Prior to COVID-19, our institution initiated a quality improvement (QI) program to improve comfort measure (CM) uptake, beginning with specific clinical areas with intent to scale up over time. When the COVID-19 vaccine was approved, mass vaccination clinics provided an opportunity to rapidly improve CM uptake across the institution. Methods: Mass vaccination clinics were staffed by nurses from across the hospital. Clinics occurred in 3 waves, based on federal approval for age groups (1: 12y+, 2: 5-11y, and 3: 6m-4y). Each wave was treated as its own PDSA cycle. Families completed a post-vaccination survey to determine what CMs were offered, how much CMs seemed to help their child, and plans for using similar CMs again. Across each wave, data on uptake were considered alongside qualitative feedback from families, nursing staff, and administrative leaders to determine targets for the next PDSA cycle. Selected interventions broadly considered the evidence base across ages, mass vaccination clinic flow, caregiver/patient understanding of available tools, and infrastructure available to support institutional change efforts. Results: Uptake of targeted CMs increased in response to PDSA waves, and generally remained stable thereafter. Across waves, families reported that CMs helped their child with pain/distress (Wave 1: 71%; 2: 88%; 3: 88%) and intended to use some or all the same CMs for future vaccinations (Wave 1: 84%; 2: 96%; 3: 97%); rates increased across both with younger patient age. Conclusions: In a fast-paced mass vaccination clinic, uptake was good to excellent across various CMs and age groups and yielded high satisfaction and interest in future use. Further, many nurses became change agents in their clinical area. Lessons learned will be discussed.
-

Objective and self-report outcomes of intensive interdisciplinary pain treatment for youth with chronic pain with and without functional neurological disorder
Kelsey Zaugg, Dustin Wallace, Kayla Friesen, and Cara M. Hoffart
Introduction: Intensive interdisciplinary pain treatment (IIPT) is effective for youth with chronic pain (CP). Many from this population also experience functional neurological disorder (FND). Treatment outcomes for patients with CP and FND during and after IIPT have not been thoroughly examined, and studies utilizing objective physical and occupational therapy measures are particularly lacking. Methods: 301 adolescents (M age=15.34 years, 84.4% girls, CP only: 187, CP and FND: 114) participated in individualized physical, occupational, psychological, and other therapies. Self-report (COPM, functioning) and objective (BOT-2, 6-minute-walk) measures were administered pre-IIPT, post-IIPT, 6-months-post, and one-year-post. Results: Overall, there were strong and statistically significant improvements from baseline to all other time points (all p’s<.01) for those with CP only and those with CP and FND. From pre-IIPT to post-IIPT, there were statistically significant group differences indicating less improvement for those with FND on coordination, strength, and agility tasks of the BOT-2 (F(1,255)=6.08, p=.014; F(1,255)=9.03, p=.003); on COPM satisfaction (F(1,258)=6.31, p=.013); and 6-minute walk test distance (F(1,217)=5.08, p=.025). Significant group differences from baseline to all follow-up timepoints indicated those with FND had less improvement on COPM performance (F(1, 258) =14.16, p <.001; F(1,150)=5.95, p=.016; F(1,96)=6.394, p=.013) and overall functional disability F(1,262)=15.43, p <.001; F(1,172)=4.250, p=.041; F(1,119)=5.44, p=.021). However, the BOT-2, 6-minute-walk and COPM satisfaction did not differ significantly at follow-ups, indicating no significant difference between those with and without FND. Conclusions: This study suggests that objective and self-report outcomes differ significantly between IIPT participants with CP and FND compared to those with only CP. However, both groups improved significantly overall, and some differences weakened in magnitude over time.
-

Prevalence of Iron Deficiency in Patients with Inherited Bleeding Disorders
Thomas Cochran, Brian Lee, and Shannon Carpenter
Background: Synthesis of hemoglobin is one of several important roles iron plays in the human body. Approximately 50% of all anemia cases may be caused by iron deficiency which is frequently caused by chronic blood loss. Patients with bleeding disorders have greater propensity for blood loss and therefore may have a higher prevalence of iron deficiency when compared to the general population. However, few studies have assessed the prevalence of iron deficiency in children with inherited bleeding disorders. Objectives: This study aims to identify the prevalence of iron deficiency in children with an inherited bleeding disorder. Methods: A retrospective analysis of children with any inherited bleeding disorder seen in Children’s Mercy Hospital’s hemophilia treatment center between 2010 and 2020 was performed. Iron deficiency was defined by recently published serum ferritin thresholds outlined by the National Health and Nutrition Examination Surveys (NHANES) [1]. Subjects were defined as iron deficient if they met these criteria at any visit. Other patient characteristics such as concomitant iron deficiency risk factors and use of iron supplementation were included in the analysis. Results: There were 507 patients with inherited bleeding disorders who were included in this analysis. Of the 177 patients who had serum ferritin collected, 103 (58%) were iron deficient. In patients who were iron deficient, 69% were female and 51% were aged 6 to 15 years. Notably, of the 68 (38%) males with serum ferritin collected, 31% were iron deficient. Of the 478 patients who had blood counts measured, 217 (45%) were anemic. Of the patients with anemia, 138 (64%) were male. The proportion of patients found to be iron deficient in each age group are displayed in figure 1. Only 4 (4%) of the patients found to be iron deficient had other iron deficiency risk factors. Furthermore, 64 (62%) of patients who were identified as iron deficient were on some form of iron supplementation. Conclusion: The results of this study suggest that iron deficiency is more prevalent in patients with inherited bleeding disorders when compared to the general population. Prior epidemiologic analysis of iron deficiency and iron deficiency anemia is mostly focused on adolescent females, but these results suggest that males with inherited bleeding disorders are at comparable risk. The actual prevalence of iron deficiency in male patients may be higher than this report suggests considering ferritin levels were not measured in 75% of males. Furthermore, the prevalence of iron deficiency is likely underreported in studies prior to the recently published NHANES physiologic ferritin thresholds. These patients represent half of the population identified for inclusion in this retrospective study.
-

Psychosocial Needs of Pediatric Patients with Cancer Predisposition Syndromes: Standardized Screening Needed
Meredith Ehrhardt, Mirae Fornander, and Rachel Moore
Introduction Cancer predisposition syndromes (CPS; e.g., Li-Fraumeni syndrome, Von Hippel-Lindau syndrome) require routine, standardized medical monitoring with accompanying unique, complex psychosocial needs (e.g., family medical needs). Despite limited research within pediatric populations, emerging literature has begun to support the benefit of routine psychosocial screening in this population. Very few pediatric CPS clinics with dedicated psychosocial care (i.e., psychology) exist in the U.S. and there is currently no standard of care for psychosocial support of CPS patients. This study aims to better understand the psychosocial needs of pediatric CPS patients and support the need for a standardized psychosocial screening protocol among pediatric CPS clinics. Methods Data collection in the Surveillance for Predispositions to Tumors (SPoT) Clinic was obtained from 2021-2022 via REDCap using standardized psychosocial screening battery for all patients and their parents which included demographic, academic, developmental, general health, quality of life, resilience, and genetic diagnosis reaction measures and suicidality, mood, and anxiety screeners. Results A total of 25 patients between 2 and 19 years old (M=11.80, SD=4.70) completed the standardized screening. Patients were predominately assigned female at birth (N=15, 60%) and non-Hispanic White (N=19, 76%). Most denied academic concerns (N=17, 68%). A subset of patients reported concerns for depression (N=5, 20%, M=5.64, SD=5.45; mild n=3, moderate n=2, severe n=1) or anxiety (N=4, 16%, M=5.50, SD=5.77; mild n=3, moderate n=1, severe n=1). Few patients reported a current or past therapy history (N=7, 28%). Patients reported medium to high levels of hope for their future (N=8, M=4.40, SD=1.23). Conclusions Preliminary, descriptive results suggest that CPS patients have medium to high levels of hope and few concerns for depression and anxiety compared to adult CPS patients. Importantly, this study describes a standardized psychosocial screening protocol model for CPS clinics as a first step toward identifying a standard of care for psychosocial screening.
-

Experiences of Racism among Black and African Children with Asthma
Esosa Adah, Rayanna Tucker, and Bridgette Jones
Rationale: The relationship between adverse childhood experiences, toxic stress and asthma risk has been previously described in the literature among adult and pediatric populations. Studies have identified experiences of racism as a chronic stressor associated with asthma morbidity however, most studies have focused primarily on adults or parental perspectives. We initiated a pilot study to characterize described experiences of racism among Black/African American children with persistent asthma and describe some of our initial results. Methods: Children were asked to complete the “Perceptions of Racism in Children and Youth,” a validated questionnaire, measuring perceptions of racism and discrimination. Responses were analyzed for frequency of shared responses. Results: Ten children ages 7-17 years completed the survey. 100% of participants(n=10) endorsed experiencing at least one racist or discriminatory event ranging in frequency from once to weekly. Overall, 60% of children endorsed “being called an insulting name”; 20% endorsed “being watched closely or followed around by security guard at a store/mall”; 20% endorsed “having the feeling someone was afraid of them”; 30% endorsed “someone making an insulting remark about one’s race ethnicity, or language”;10% endorsed “seeing one’s family being treated unfairly due to race, skin color, accent or culture differences.” Conclusions: Initial data from this ongoing pilot study demonstrates that children with asthma endorse a broad range of experiences of racism. Intentional efforts are needed to raise awareness of racism as a chronic, toxic stressor experienced by children with asthma and efforts should be made to mitigate the impact on asthma morbidity.
-
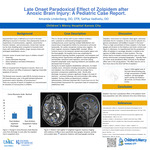
Late Onset Paradoxical Effect of Zolpidem after Anoxic Brain Injury: A Pediatric Case Report
Amanda Lindenberg and Sathya Vadivelu
Case Diagnosis Acquired brain injury (ABI) is a common cause of disability. It is defined as an insult to the brain that subsequently results in impairments of communication, cognition, sensorimotor function and behavior. Anoxic brain injuries can be particularly disabling. A common cause of a anoxic injury in children is cardiopulmonary arrest. The revised coma recovery scale (CRS-R) is a standardized assessment for level of arousal after brain injury. Case Description A 16 year old healthy boy was admitted after an unwitnessed pulseless cardiopulmonary arrest requiring 3 defibrillations and 2mg of epinephrine. His cardiac and genetic workup was negative. His brain imaging revealed diffuse ischemic injury to the temporal, parietal and occipital regions and the basal ganglia. His hospital course was complicated by dysautonomia requiring clonidine, propranolol, and lorazepam, hypertonicity and agitation requiring gabapentin and baclofen, botox injections, and serial casting, impaired sleep/wake cycles requiring melatonin and zolpidem, and decreased level of arousal. He was admitted to inpatient rehab from February 26 to April 5, 2016. He was trialed on multiple neuro-stimulants including zolpidem but did not emerge from a persistent vegetative state. Shortly after discharge, his parents reported a change in his level of alertness and command following after administration of zolpidem. During a follow up appointment, a trial of zolpidem confirmed improved CRS- R score from 6 to 14. Discussions Zolpidem is medication that stimulates the ω-1 site of a gamma aminobutyric acid (GABA)A receptor in the brain. The basal ganglia and striatum to the thalamus and motor cortices are abundant in these receptors. Moreover, it is hypothesized that the paradoxical effect of zolpidem in neurologic disorders is secondary to the agonism. There is a paucity of reports in children. Conclusions Zolpidem may have a paradoxical effect in the pediatric brain injury population after a prolonged period of medication.
-
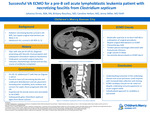
Successful VA ECMO for a pre-B cell acute lymphoblastic leukemia patient with necrotizing faciitis from Clostridium septicum
Johanna I. Orrick, Brittany Rouchou, Cara Holton, and Jenna Miller
REPORT: It can be challenging to calculate the risks versus benefits of a potential Extracorporeal Membrane Oxygenation (ECMO) pediatric candidate. A patient with an oncologic comorbidity and an increased potential of needing near-future surgical interventions carries higher ECMO risks. We report a successful VA ECMO run of a newly diagnosed acute lymphoblastic leukemia (ALL) adolescent with Clostridium septicum necrotizing fasciitis. A previously healthy 16-year-old male was diagnosed with pre-B cell ALL after having an ileocolic intussusception and a Clostridium septicum blood infection. He underwent successful surgical intussusception reduction, started induction chemotherapy, and completed a ten-day course of antibiotics. On hospital day (HD) 15, he experienced significant abdominal pain and profound septic shock. His abdominal CT scan was concerning for air and fat stranding within the anterior abdominal wall. He was taken to the OR, where he was found to have necrotizing fasciitis and underwent debridement and silo placement. He returned to the Pediatric Intensive Care Unit on multiple high-dose vasoactive infusions with hemodynamic instability refractory to aggressive volume resuscitation. His instability and lactic acidosis progressed quickly in the 60 minutes after arriving from the OR, and thus, careful consideration was given to his ECMO candidacy. The prognosis for survival from ALL is 60-90% in adolescents (1,2). Repeat blood cultures had not speciated at the time of our patient’s hemodynamic decline, but necrotizing fasciitis survival in pediatrics ranges from 60-90% (3-6). Early surgical intervention increases the survival rate of myonecrosis, but future interventions would be complicated by anticoagulation on ECMO (7-9). Little more than a few case studies have been reported on the outcomes of ECMO patients with active necrotizing fasciitis (10,11). Compared to other pediatric ECMO patients, immunocompromised and oncological patients on ECMO have historically higher mortality rates. However, current survival reports have been improving (12-15). Ultimately, he was determined to be an ECMO candidate. While the team mobilized, he suffered a 16-minute bradycardic arrest but was successfully cannulated to VA-ECMO. Bivalirudin was used in anticipation of surgical procedures considering its short half-life. Chemotherapy was held, and antimicrobials were broadened. Repeat surgical debridement happened on ECMO day 2. Heparin neutralized partial thromboplastin time (PTT) measured at 69 seconds, so no anticoagulation adjustments were made for the procedure. He received alternating 1.1x plasma exchanges and granulocyte infusions during the first week of ECMO. A large volume GI bleed complicated his ECMO run, but he was able to be successfully decannulated on ECMO day 8. He was discharged home on HD 54 neurologically intact and is currently in the Interim Maintenance I stage of his pre-B ALL treatment. He has been home for six months at the time of this report. (Discharged 5/11/2022) While more centers are reporting their individual ECMO experiences in high-risk oncologic pediatric populations, the data is lacking for pediatric ECMO cases with multiple comorbidities and surgical intervention needs while on ECMO. Our approach required surgical collaboration and would be necessary for any future patients such as this. ECMO candidacy should not be ruled out for these patients, and case-by-case candidacy determinations should be undertaken. Understanding outcomes of the underlying disease and acute processes could improve with increased data collection and sharing.
-

Philippines Global Health
Thomas Rieth, Kourtney Bettinger, and Ryan Northup
Provides reflections on resident's experience working with cardiologists and neonatologists in Manila.
-

San Pedro Hospital - San Pedro La Laguna, Guatemala
Jennifer Paumen
Primary objective: to learn about the healthcare system of Guatemala including the challenges and strengths.




